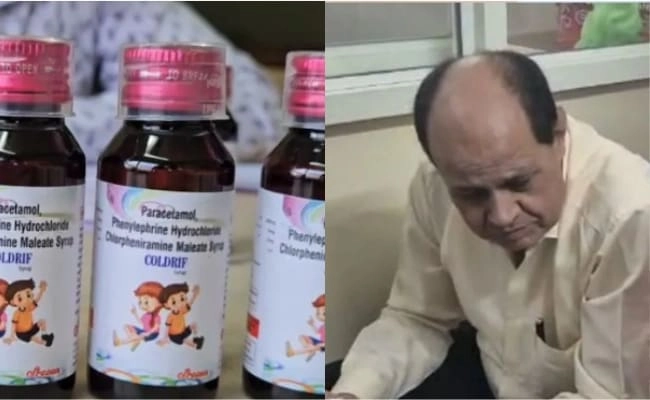
The recent tragedy involving the ‘Coldrif’ syrup, which has been linked to the deaths of eleven children in Madhya Pradesh, has sent shockwaves through the medical community and the public alike. The syrup, reportedly used to treat cough and cold symptoms in pediatric patients, has raised serious concerns about its safety and the regulatory oversight of medications prescribed to children. As investigations continue, the focus has shifted to the healthcare professionals involved in prescribing the medication, leading to the arrest of a local doctor who had prescribed ‘Coldrif’ to several of the deceased children.
The incident highlights the critical importance of ensuring that medications, especially those administered to vulnerable populations like children, are rigorously tested for safety and efficacy. The deaths have sparked outrage among parents and community members, who are demanding accountability and transparency from both healthcare providers and pharmaceutical companies. This tragedy has also ignited discussions about the need for stricter regulations concerning the approval and monitoring of over-the-counter medications, particularly those marketed for pediatric use.
In light of these events, public health officials are urging parents to exercise caution when administering medications to their children and to consult with healthcare professionals about any potential risks associated with specific products. Moreover, the healthcare community is being called upon to reinforce protocols for prescribing medications, ensuring that they are based on the latest evidence and guidelines. The fallout from this incident is likely to lead to increased scrutiny of drug safety practices and a renewed commitment to protecting the health and well-being of children. As the investigation unfolds, the hope is that lessons learned will prevent similar tragedies in the future.